Bluenote Raises $10M from Lux Capital & Elad Gil to Transform Life Sciences Workflows with AI

Bluenote’s mission is to bring life-saving medicines, medical devices, and diagnostics to patients & clinicians sooner by streamlining regulatory and compliance workflows.
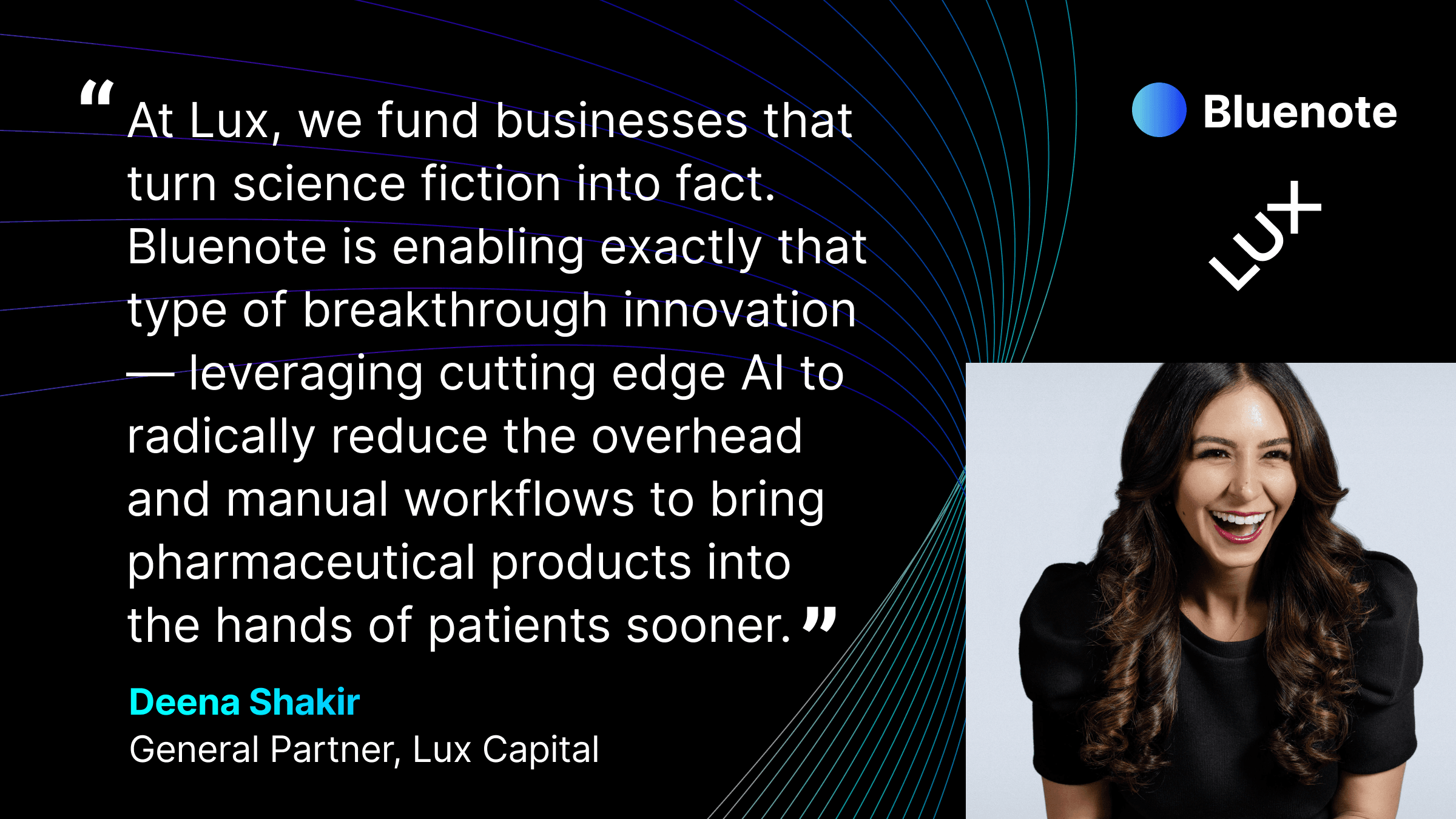
Bluenote Raised Financing from Leading Investors in AI & Biopharma
The round is led by Lux Capital, with participation from Elad Gil, Anthropic & Menlo Ventures Anthology Fund, McKesson Ventures, Avichal Garg/Electric Capital, Moxxie Ventures, Carbon Silicon Ventures, and leaders in AI and life sciences — Othman Laraki (CEO Color Health), Fidji Simo (CEO Instacart, Co-founder Metrodora Institute, OpenAI Board), Mike Nohaile (CEO Prellis Biologics, previously Amgen & Novartis Executive), Kristen Fortney (CEO BioAge), Eric Morgen (COO BioAge), Qasar Younis (CEO Applied Intuition), Linus Upson (Verily), and Jeffrey Low (Life sciences investor).
Bluenote’s AI Platform Streamlines Regulatory & Compliance Workflows
Companies in the life sciences industry are heavily regulated, and need to invest significant time and resources to fulfill their regulatory and compliance obligations. While the regulatory framework is in place to ensure patient safety, clinical efficacy, and consistent product quality, the work required to produce compliance documentation is time-consuming and largely manual.
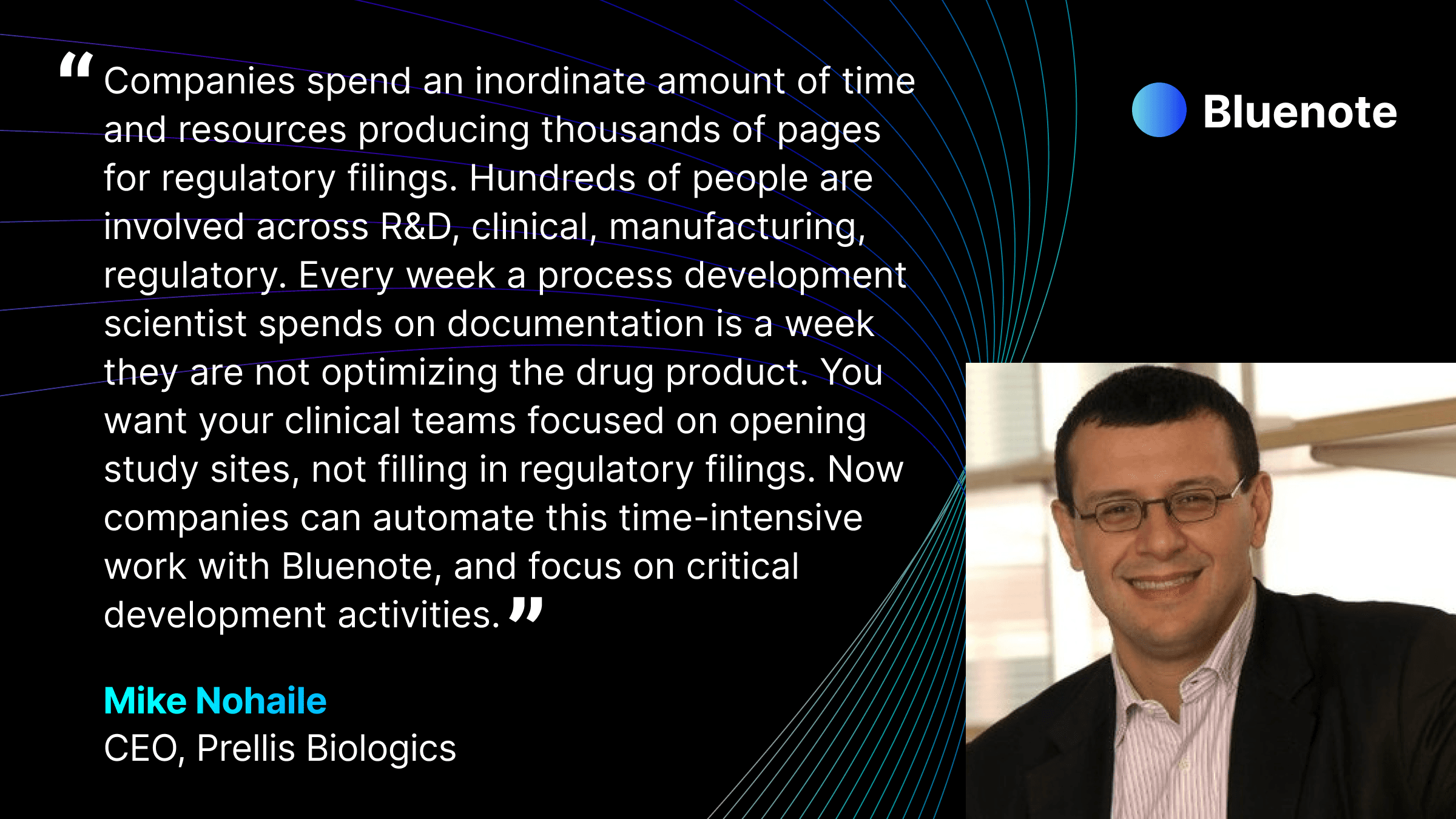
Following a clinical trial’s completion, companies typically spend 8-9 months preparing regulatory submissions1. Across the industry, thousands of scientists, engineers, and development professionals spanning multiple functions — research, clinical, manufacturing, regulatory and quality — dedicate significant time to produce thousands of pages for regulatory filings and manage complex interdependencies between protocols, technical reports and compliance requirements. This time could instead be redirected towards scientific research and product development to bring new breakthroughs to patients.
Bluenote has developed a generative AI technology platform for life sciences companies to streamline regulatory workflows and bring their breakthroughs to patients sooner. Today, the platform’s most widely used applications focus on producing regulatory filings, technical reports, protocols, Standard Operating Procedures (SOPs), validation reports, risk analyses, manufacturing documentation and more. Bluenote has deployed 15+ applications for scientists, engineers, quality, regulatory and manufacturing teams. Bluenote has developed fine-tuned models that are 90% preferred over off-the-shelf models. The company is rapidly expanding the number of applications on its platform.

“Life sciences leaders are moving quickly to deploy generative AI across dozens of workflows. In this industry, every day counts. Each day a breakthrough therapy or device is not in the hands of clinicians and patients is a lost opportunity to save lives,” said Fatima Sabar, Bluenote Co-founder & CEO. “Bluenote’s AI agents are being integrated into laborious workflows to accelerate time to market and increase operational excellence.”
Bluenote has Developed a Secure Generative AI Platform that is Tailored to the Life Sciences Industry:
- Accuracy and verifiability of AI outputs
- Bluenote’s product is engineered to include only factual details from traceable primary sources, combining guardrails with custom Large Language Models (LLMs), and inserting call-to-action placeholders for human reviewers to provide details where additional context is needed.
- Customized outputs via proprietary datasets, connectivity to data lakes & a multi-model approach
- Bluenote developed domain-optimized Retrieval-Augmented Generation (RAG) that ingests data from various data lakes and third party applications, accurately processes the datasets, including industry-specific edge cases, and indexes them to support unique application requirements.
- Bluenote finds that there is no one-size-fits-all model, and instead, combines the best models from Anthropic, OpenAI, Google, and its own fine-tuned models based on proprietary datasets. With new models regularly entering the market, Bluenote continuously evaluates and updates model configurations to ensure that customers are among the first to benefit from the latest advances.
- Single, secure platform that powers multiple workflows and functions
- There are compounding benefits to having a single platform running AI applications instead of multiple vendors for each. The same proprietary knowledge base is used for multiple applications. The outputs from one workflow become inputs into another, enabling the propagation of changes. In addition, customers prefer to consolidate their proprietary data within a single, secure environment for simplicity.
With the new financing, Bluenote is rapidly expanding its platform to support additional mission-critical, time-intensive workflows, and enabling life sciences companies to bring their breakthroughs to patients sooner.
1 Getting strategic about new-product submissions in the pharma industry.
